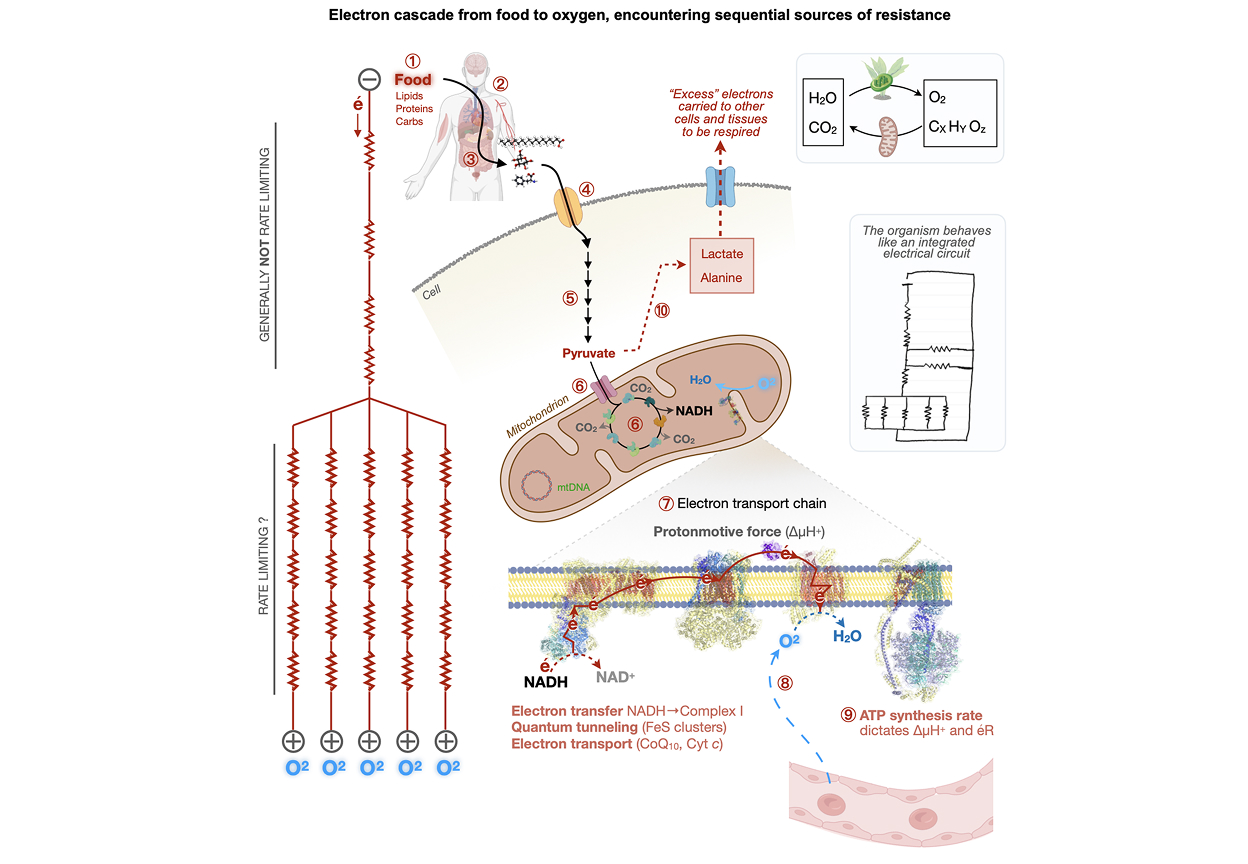
The ERP describes how the energy transformation that sustains our lives and everything we experience requires energy resistance, éR. But also, how excessive éR is the common basis of inflammation, aging, disease, and aging. We show how the cytokine GDF15 is a marker of éR, drawing from a database of >50,000 people to provide an initial validation of the model. The ERP draws from the Power law to propose a simple formula, a preliminary quantitative framework to help us think quantitatively about how energetic processes and substrates interact with the flux of electrons through mitochondria and the rest of our biological circuitry, to produce variable levels of éR that is sensed by the organism and shape our health.
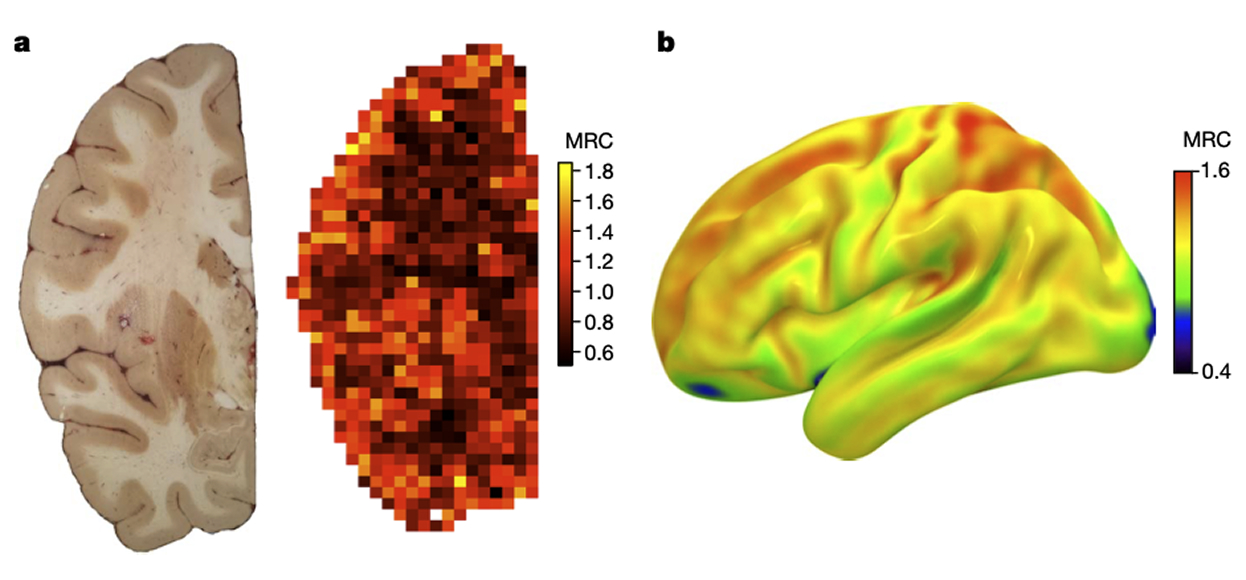
The MitoBrainMap v1.0 is a systematic description of mitochondrial quantity and diversity across the human brain, and between different brain cell types. Its predictive algorithm provides a brain-wide probabilistic map of molecular and enzymatic mitochondrial features in the standard neuroimaging space used around the world.
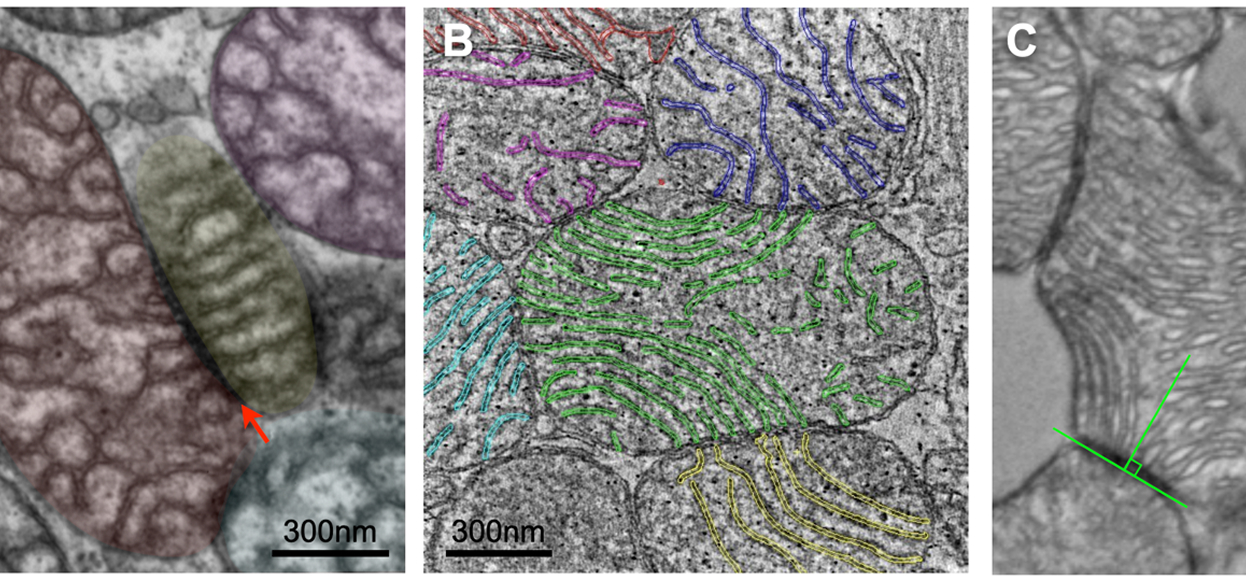
This study provided the first physical evidence that mitochondria exchange information with one another. The alignment of cristae (the inner membrane allowing mitochondria to respire) across energized mitochondria suggests the existence of invisible field(s) organizing the biophysical structures within and across the mitochondrial collective.
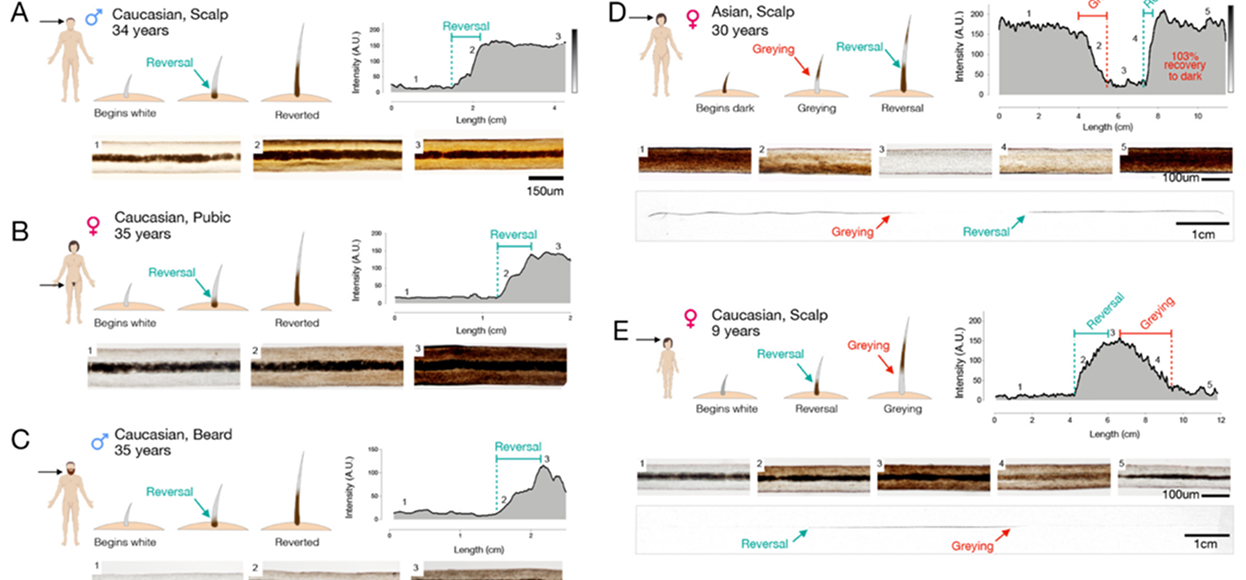
Inspired to understand why some people age faster and others more slowly, we turned to the heterogeneity of hair greying. We then serendipitously discovered that hair greying is reversible, and that grey hairs exhibit a signature of mitochondrial recalibration, linking life stress, energy metabolism, and hair greying for the first time.
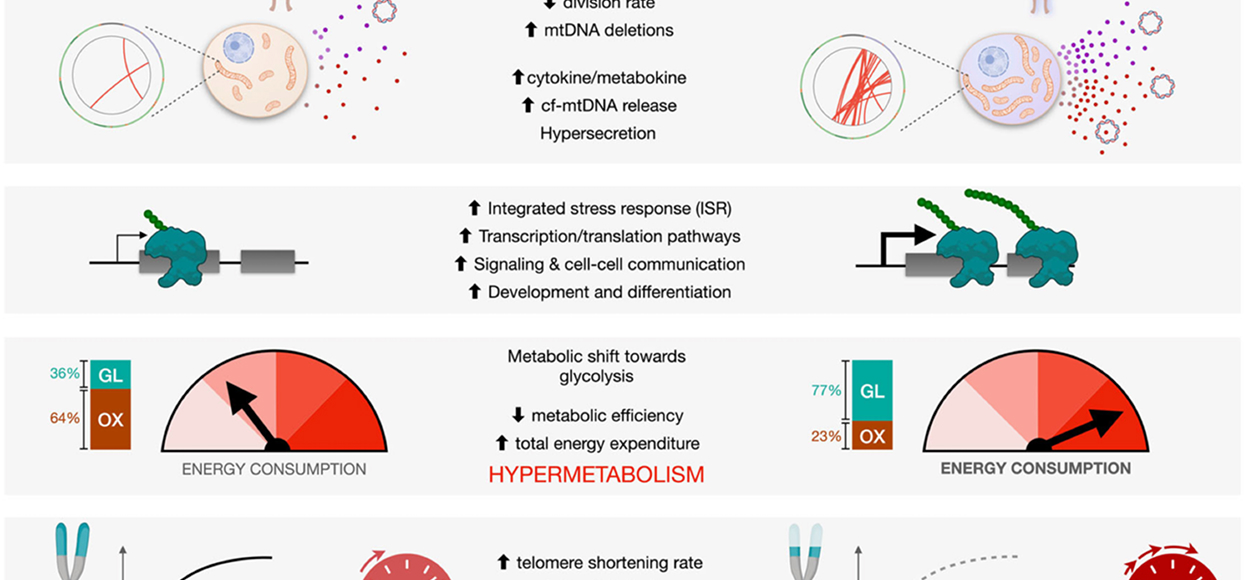
The Cellular Lifespan Study examined cellular energetics and aging trajectories over up to nine months in culture. This study showed that 1) mitochondrial oxidative phosphorylation (OxPhos) defects cause hypermetabolism (increase the cost of living), 2) OxPhos defects accelerate cellular aging (telomere shortening and epigenetic clocks), 3) fragments of the mitochondrial genome are spontaneously inserted de novo into the nuclear genome over the course of the cellular lifespan (Zhou et al. Plos Biol 2023), and 4) as cells age and activate senescence stress responses, they consume more, not less energy. Similar studies in this system revealed similar hypermetabolic and accelerated aging effects of chronic glucocorticoid signaling (Bobba-Alves et al, PNEC 2023).
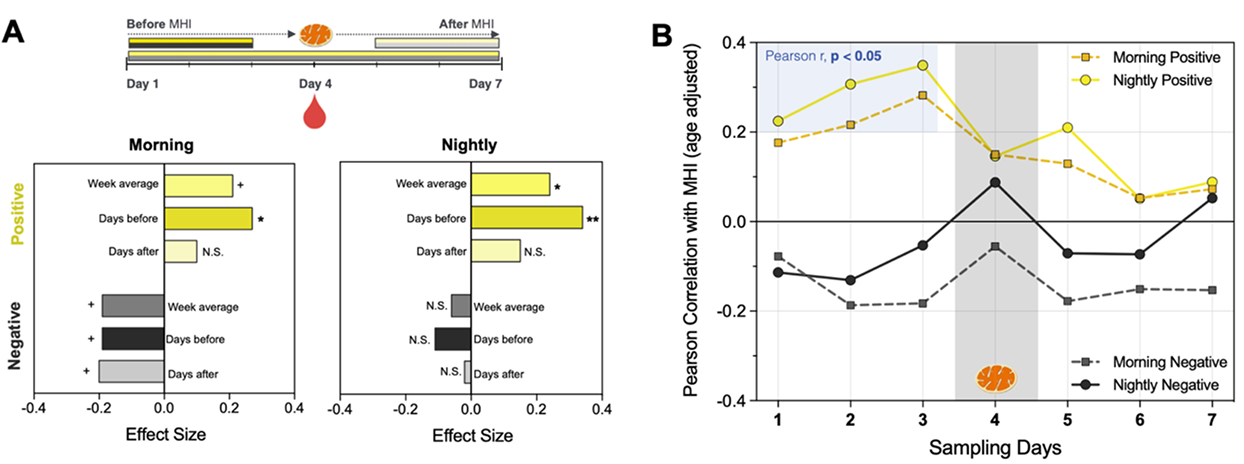
The Mitochondrial Health Index (MHI) study was the first to document a directional link between mood and mitochondrial energy transformation capacity in immune cells. We have since learnt how different immune cells have unique mitochondria (Rausser et al. eLife 2021). This first-generation laboratory tool to quantify mitochondrial energy transformation capacity evolved into the second-generation Mitochondrial Respiratory Capacity (MRC).
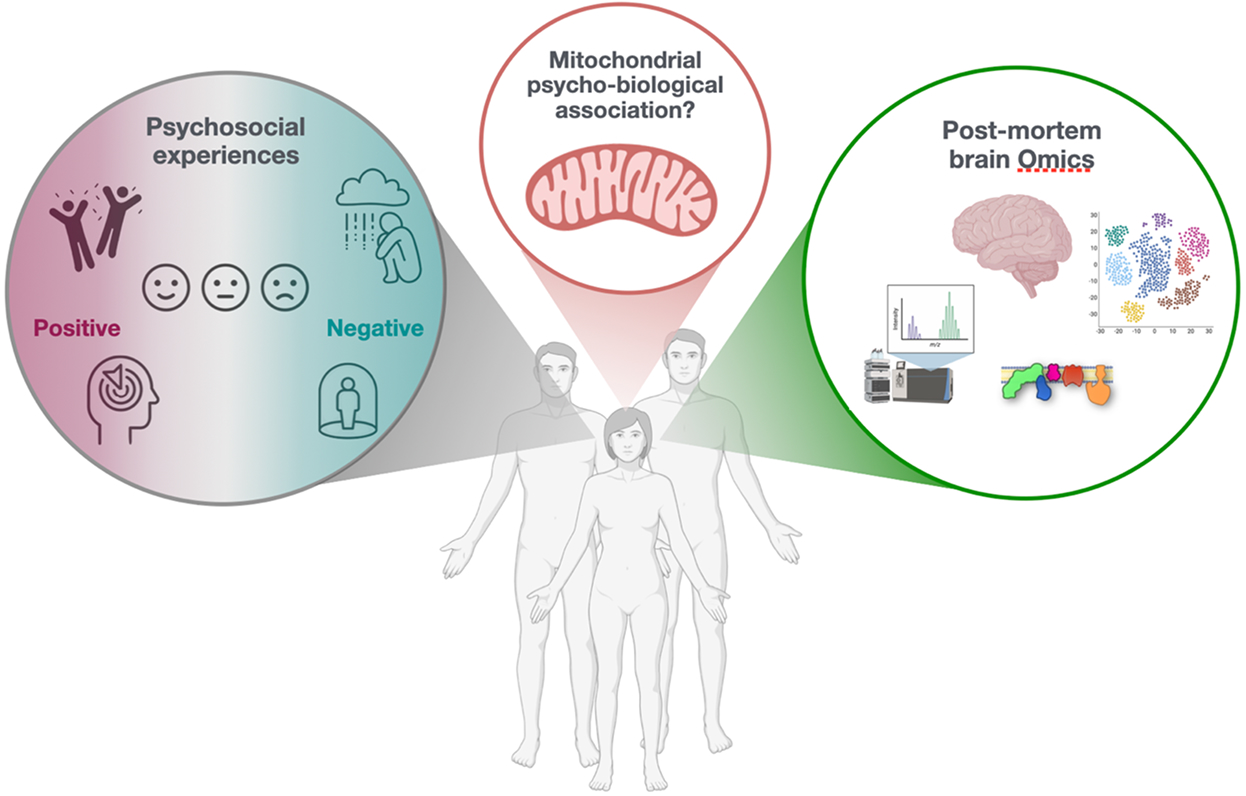
Here we showed that the biology of brain mitochondria (specifically, mitochondrial electron transport chain complex I) is associated with positive and negative psychosocial factors. In single nucleus RNA sequencing data, the strongest associations emerged in glial cells, not in neurons, suggesting new avenues for understanding the basis of human experiences beyond classic neuron-centric models.
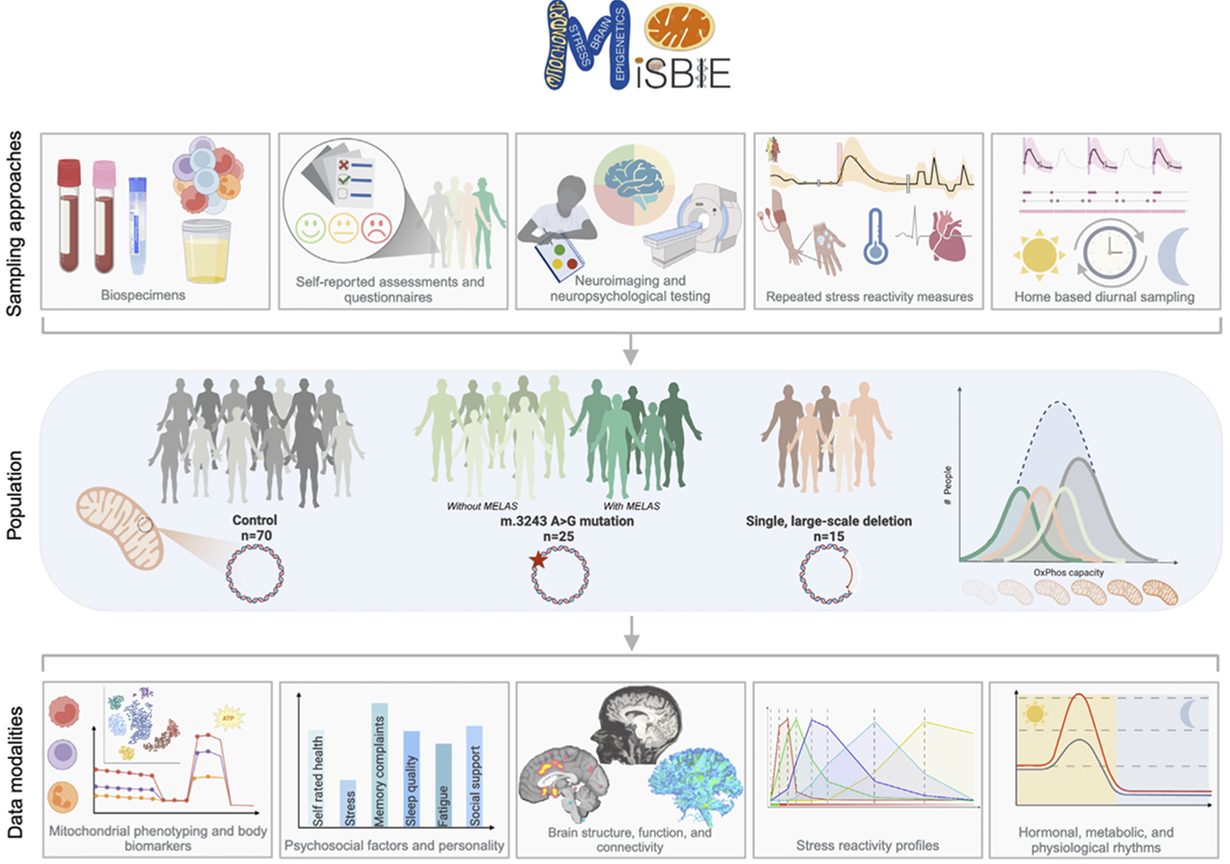
The Mitochondrial Stress, Brain Imaging, and Epigenetics (MiSBIE) study is a deep-phenotyping study of individuals with a wide spectrum of mitochondrial health. Data from MiSBIE have led to several discoveries around the mind-mitochondria connection, the role of mitochondria in shaping stress responses, and defined the novel status of “stress hormones” for the classic metabolic cytokines/metabokines FGF21 (Kurade et al. Nat Metab 2025) and GDF15 (Huang et al. Mol Genet Metab 2025).